 | ||
| || home ||| units ||||| help | ||
| All Units | > | Unit 11 - Air pressure | > | Investigation 2 - Air pressure and the lungs | > | Trial 3 |
Trial 3 - How much can you exhale?
-
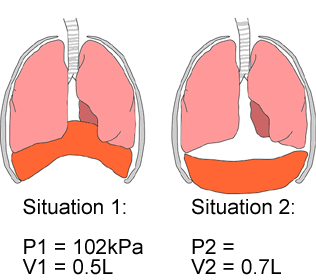
When you increase the size of your chest cavity, the pressure in your chest cavity goes down. It turns out that for any fixed amount of air, the relationship between pressure (P) and volume (V) is defined as P1V1 = P2V2. The "1" stands for initial values and the "2" stands for final values. This relationship is called Boyle's Law. Using Boyle's Law, can you calculate what the pressure in the chest cavity is in situation 2 below?

-
With the pressure lowered in your chest cavity, what happens to your lungs?

-
Using one breath, blow up a balloon as big as you can. Measure the diameter of the blown up balloon and record it. One way to measure the diameter is to wrap a piece of string snugly around the balloon and then measure the wrapped length of the string with a meter stick.
Diameter:

-
Knowing that the equation for the volume of a sphere is:
volume of sphere = 4/3πr3
where π = 3.1415
and r = 1/2 of the diameter you measured above,
What is the approximate volume of air in the balloon?

-
Is this a good way to estimate the amount of air you exhaled? Why or why not?

-
Using your inflated balloon, can you come up with a more accurate way of figuring out the amount of air you exhaled? Write your ideas below. Be prepared to share your ideas with the class.

-
Compare your ideas with the ideas of your classmates. As a group, can you come up with ideas for making a more accurate measurement of the amount of air someone can exhale?
 |  |  |
Copyright 2005 The Concord Consortium, All rights reserved.